- Who We Are
- Clinician Employment
- Publications
- Witness to Witness (W2W)
- Kugel & Zuroweste Health Justice Award
- Your Voice Matters: Photovoice Project
Wed, 02/24/2021 | by MCN Admin

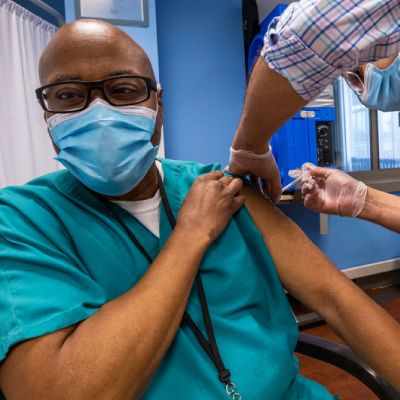
[Editor’s Note: This article was first published on Healio.com. Laszlo Madaras, MD, MPH, Migrant Clinicians Network’s Chief Medical Officer, is one of the co-authors. Here, we reprint the article with permission from Healio and present it for the first time translated into Spanish. Read the original article on Healio.com.]
By Raghavendra Tirupathi, MD, FACP; Laszlo Madaras, MD, MPH, FAAFP; Ali Rabaan, PhD; and Prasannalaxmi Palabindela, MD
The CDC estimates that, as of Jan. 5, more than 4.8 million people in the United States have received the first dose of a COVID-19 vaccine.
While many health department and vaccine clinic systems for vaccine distribution are built for the general public, they often miss vital elements that make vaccines easily accessible to RIM communities. The federal government is committed to ensure that safe, effective, cost-free vaccines are available to the entire US public, regardless of their immigration status, however, RIM communities are still finding it more difficult to access vaccines than the general public, and vaccine dissemination sites may unknowingly be erecting unnecessary barriers.
In addition to those barriers already mentioned, RIM communities often struggle with restricted mobility, geographic isolation, limited digital access (preventing online sign-up for vaccine appointments), low levels of literacy and formal education, limited English proficiency, lack of transportation, and fear spurring from immigration status and job loss that is antagonized by excessive requests for personal information and documentation. These barriers are in addition to the regular hesitancy for the COVID-19 vaccine that some of the general US public has, as well as additional fears due to the negative history of vaccines and communities of color in the US. In light of these significant and overlapping barriers, many vaccine dissemination sites are inadequately prepared to assist RIM community members with accessing vaccines.
During a recent event, President-elect Joe Biden said his administration will increase the pace of COVID-19 vaccine distribution, with a goal of delivering 100 million doses during his first 100 days in the White House.
As vaccines continue to be rolled out, primary care providers are preparing their patients for vaccination. In this article, Infectious Disease News Editorial Board Member Raghavendra Tirupathi, MD, FACP, FIDSA, medical director at Keystone Infectious Diseases/HIV, chair of infection prevention at Summit Health and clinical assistant professor of medicine at Penn State College of Medicine; Laszlo Madaras, MD, MPH, FAAFP, medical director of educational affairs at WellSpan Summit Health Campus, chief medical officer at Migrant Clinicians Network, clinical assistant professor of medicine at Penn State College of Medicine and adjunct assistant professor of family medicine at Brown University; Ali Rabaan, PhD, head of the Molecular Diagnostic Laboratory at Johns Hopkins Aramco Healthcare in Saudi Arabia; and Prasannalaxmi Palabindela, MD, hospitalist physician at Jennie Stuart Medical Center in Hopkinsville, Kentucky, answer common questions about COVID-19 vaccination.
Q: What is an mRNA vaccine?
A: mRNA vaccine is new type of vaccine technology compared to conventional vaccine approaches with high potency, capacity for rapid development and potential for low-cost manufacture and safe administration. mRNA is the intermediate step between the translation of protein-encoding DNA and the production of proteins by ribosomes in the cytoplasm. The vaccine has pieces of mRNA encased in a lipid shell, which provide the genetic code for our cells to produce viral proteins. Once the proteins, which don’t cause disease, are produced, the body launches an immune response against the virus, enabling the person to develop immunity.
There are two COVID-19 vaccines that have received FDA emergency use authorization: the Pfizer-BioNTech vaccine (16 years and above) and the Moderna vaccine (18 years and above).
Q: What is the schedule for the vaccines?
A: The vaccine schedule for the Pfizer vaccine is two doses administered intramuscularly (IM) 21 days apart. The Moderna vaccine schedule is two doses IM 28 days apart.
Both doses are necessary for protection. Efficacy of a single dose has not been systematically evaluated.
Q: Can you get COVID-19 from the vaccine?
A: mRNA vaccines do not contain a live virus and do not carry a risk of causing disease in the vaccinated person. The mRNA in the vaccine does not code for the virus but only the spike proteins used to mount an immune response.
Q: How effective is the vaccine in preventing disease?
A: Both vaccines are known to prevent disease with a high efficacy rate of up to 95% in phase 3 trials. To put this in perspective, the efficacy is almost as good as the measles vaccine, which is historically known to be the most effective vaccine.
Q: Is one mRNA vaccine better than the other vaccine in terms of efficacy and safety?
A: Both Pfizer and Moderna vaccines are equally efficacious and safe. Pfizer has more stringent storage requirements (-70°C/-94°F) but that should not be a reason for not offering the vaccine.
Q: Can mRNA vaccines alter the genetic code of an individual?
A: The human cytosol efficiently clears the mRNA after the process of translation into spike proteins. The mRNA does not enter the nucleus or alter the human genetic code.
Q: Can mRNA vaccines result in a positive COVID-19 test?
A: It is not possible for someone to have a positive COVID-19 test from the vaccine, but if a person acquires infection immediately prior to or within the 14 days of vaccination, he or she could have a positive test due to infection and not vaccination.
Q: Is routine testing needed prior to receiving the COVID-19 vaccine?
A: No. Routine COVID-19 PCR or serology testing is not needed before or after vaccination unless indicated for other purposes.
Q: Do you need to follow mitigation measures after receiving the vaccine?
A: Vaccines are being administered in a staggered fashion due to limited availability, with health care workers and long-term care facility residents first in line, currently. Hence, there is still a large susceptible, nonimmunized population yet to be vaccinated. We also don’t know from the data available so far if the vaccine prevents infection, even though it is very efficacious in preventing disease.
Trials were designed to look at preventing symptomatic disease. It is theoretically possible that the vaccines also prevent infection. Until we have convincing data and a substantial portion of the population is immunized, the recommendation is to continue all mitigation measures, including masking, social distancing, hand hygiene, avoiding crowds, following CDC travel guidance, following quarantine guidance after an exposure to someone with COVID-19 and following any applicable workplace or school guidance.
Q: What are the common adverse events with the vaccines?
A: The most common adverse effects noted in trial participants were fatigue, headache and fever. No serious adverse effects were noted in trial participants for the duration of follow-up. Post-vaccination symptoms were seen more frequently following the second dose.
Symptoms consistent with COVID-19 in the first 7 to 10 days post-vaccination (cough, shortness of breath, loss of taste and smell) should need further evaluation as these could be signs of active infection. It would be prudent to educate the vaccinees regarding the expected symptoms and encourage them to report serious symptoms on V-safe.
Antipyretic or analgesic medications may be taken for treatment of post-vaccination symptoms. Routine prophylaxis for the purposes of preventing symptoms is not recommended at this time due to lack of information on the impact of use on vaccine-induced antibody responses.
Q: When might these symptoms most likely occur?
A: Vaccinees might develop symptoms within the first 3 days after the receipt of vaccination, and symptoms should last less than 2 days.
Q: What happens if the vaccinee misses the 21-day (Pfizer) or 28-day (Moderna) mark for the second dose?
A: Vaccinees should be encouraged not to miss the second dose and should have reminders sent to them to stay on schedule. The grace period is +/- 4 days before or after the stipulated second dose date. However, if that does happen, the first dose does not need to be repeated and catch up with second dose should be pursued at the earliest opportunity.
Q: Can different mRNA vaccines be interchangeable?
A: At this time, the CDC recommends not to interchange the two vaccines for first and second doses. The second dose of the vaccine should be of the same type and brand as the first dose.
Q: What if a patient recently had the flu or received other vaccines?
A: You should wait 14 days after having received the flu shot to get the COVID-19 vaccine. Also, any other vaccinations should be spaced out 14 days before or after COVID-19 vaccination.
Q: What if a patient previously had COVID-19, symptomatic or asymptomatic?
A: It is recommended that you still receive the COVID-19 vaccine. However, CDC and the Advisory Committee on Immunization Practices are recommending deferring vaccination among patients who have tested COVID-19 positive in the last 3 months in sites where there are not enough doses to help vaccinate all qualifying recipients. This, however, does not apply if you work in a facility which has adequate doses for all employees.
Q: What if a patient currently has COVID-19?
A: It is recommended that these patients should recover from their acute symptoms and the vaccine be administered after the period of recommended convalescence and isolation.
Q: What if someone received monoclonal antibodies or convalescent plasma for treatment of COVID-19?
A: It is recommended to wait at least 90 days from receipt of treatment to vaccinate.
Q: What are the recommendations for immunocompromised patients?
A: Immunocompromised patients were not well-represented in the original trials. Hence, no safety and efficacy data are available in these special high-risk populations. There is no recommendation from federal agencies other than having an informed discussion of risk and benefits as well as the fact that immune response to vaccination might be blunted. However, vaccination can still be considered depending on the risk of COVID-19 acquisition in a given individual.
Q: What are the recommendations for women of reproductive age, pregnant or breastfeeding?
A: No official safety or efficacy data are available from the trials as they did not look at this special population. A pregnancy test is not needed before administering the vaccine in women of reproductive age. mRNA vaccines are not live-virus vaccines and, hence, virus will not be transferred trans-placentally or through breast milk.
The American College of Obstetricians and Gynecologists, CDC and other national organizations are recommending having an informed discussion with the patient and pursuing shared decision-making based on available data regarding the level of activity of the virus in the community, the potential efficacy of the vaccine, the risk and potential severity of maternal disease, including the effects of disease on the fetus and newborn, and the safety of the vaccine for the pregnant patient and the fetus.
Pregnant women who experience fever following vaccination should be counseled to take acetaminophen, as fever has been associated with adverse pregnancy outcomes.
Q: What if the vaccinee has a prior history of food allergies or allergies to vaccine components?
A: History of severe allergic reaction (eg, anaphylaxis) to any component of the COVID-19 vaccine (like polyethylene glycol) is a contraindication to vaccination. Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the vaccine. Patients with severe allergic reaction to any vaccine or injectable therapy (intramuscular, intravenous or subcutaneous) OR anaphylaxis should be observed for 30 minutes after vaccination to monitor for the occurrence of immediate adverse reactions. All other patients should be watched for 15 minutes.
Q: Why were the vaccine trials and approval so rapid compared with other vaccines?
A: While mRNA vaccines may be a relatively new development, there is more than 3 decades of experience with mRNA vaccines. mRNA technology has been explored to develop vaccines for flu, Zika and Ebola in the past. Vaccine technology is itself 10 years old. The relatively fast trials and approval are a testament to the scientific advances of several decades and significant financial investment by multiple countries into their development. Fast pace does not mean corners were cut. The entire process was vetted by two independent committees without conflicts of interest, including the Data Safety and Monitoring Board of the trial and the Vaccines and Related Biological Products Advisory Committee, the advisory committee of the FDA.
Q: What is the recommendation for persons with recent known SARS- CoV-2 exposure?
A: If you are on quarantine, defer vaccination until the quarantine period has ended to avoid exposing health care personnel or other persons during the vaccination visit.
Q: In the trials, were people followed for long enough after COVID-19 vaccine administration?
A: Most witnessed adverse events to the vaccine happened within hours of administration, some in days and, rarely, in weeks following vaccine. The patients in the trials were followed for a total of 2 months.
Q: How long after the second dose is one protected from COVID-19?
A: Vaccine-induced immunity is robust after 14 days of the second dose. This does not mean you can let your guard down completely. Patients still need to continue masking and following all other precautions until directed otherwise by public health authorities.
Q: How long does the protection last?
A: Because the vaccines are new, this is not yet known for sure. Prediction is that the protection could last at least for a few years.
Reference
CDC. COVID-19 Vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations. Accessed Jan. 4, 2021.
CDC. COVID-19 Vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations. Accessed Jan. 4, 2021.
Like what you see? Amplify our collective voice with a contribution.
Got some good news to share? Contact us on our social media pages above.
Return to the main blog page or sign up for blog updates here.