- Who We Are
- Clinician Employment
- Publications
- Witness to Witness (W2W)
- El Premio Kugel & Zuroweste a la Justicia en la Salud
- Your Voice Matters: Photovoice Project
Low Literacy Diabetes Educational Materials/Handouts
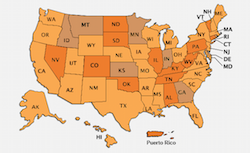
National, State, and Urban Area Vaccination Coverage Among Children Aged 19--35 Months, United States, 2005
Findings from the 2005 NIS include nationwide increases in coverage with >=3 and >=4 doses of pneumococcal conjugate vaccine (PCV) and continued high levels of coverage for the other recommended vaccines and vaccine series. In addition, no racial/ethnic disparities in coverage estimates were observed in the 4:3:1:3:3:1§ vaccine series, the recommended series for children aged 19-35 months that includes DTP/DT/DTaP; poliovirus vaccine; measles, mumps, and rubella vaccine (MMR); Haemophilus influenzae type b vaccine; hepatitis B vaccine; and varicella vaccine.
Interim VIS for Shingles
On September 11, CDC released an interim VIS for shingles
(herpes zoster) vaccine. ACIP has not yet voted on
recommendations for zoster vaccine. The interim VIS is based
primarily on information from the manufacturer's package insert.
The final VIS will be produced after the ACIP recommendations
have been published in MMWR. The final VIS could differ from the
interim version.
Send Pests Packing Comic Book
The English educational comic book was developed by Wake Forest University and offers practical preventative tips to keep unwanted pests away from your home. To view the Spanish version, search for the "Digale Adios a las Plagas Comic Book."
ACIP Provisional recommendations for prevention of Varicella
In June 2005 and June 2006, the ACIP made policy changes for use of live, attenuated varicella containing vaccines for prevention of varicella.
FreedoMed - Toll Free: 1-888-722-7556
Medical supply company, that provides low cost or totally free diabetic supplies to diabetics who are eligible through Medicare or private insurance.
RX Outreach
Easy and affordable way for people of all ages to get medicines they need. They have a list of all generic drugs you fill out the application on line or download it.
Health Network Consent Form, Spanish
CDC releases revised interim Tdap VIS. July 17, 2006
CDC released a revised interim Vaccine Information Statement (VIS) for Tdap vaccine. It is identical to the previous interim version except for minor changes in Section 3 that reflect ACIP's evolving recommendations regarding use of Tdap during pregnancy.
AHA and ACC recommend Influenza vaccination for patients with Cardiovascular disease
On May 16, 2006, the American Heart Association (AHA) and the
American College of Cardiology (ACC) published guidelines for
secondary prevention for patients with coronary and other
atherosclerotic vascular disease.
North Carolina FW Birth Defect Report
Assessment of Maternal Occupational Pesticide Exposures during Pregnancy and Three Children with Birth Defects: North Carolina, 2004. Occupational and Environmental Epidemiology Branch Division of Public Health, North Carolina Department of Health and Human Services, Raleigh, North Carolina
May 18, 2006
Ethics in Research: Protecting Human Subjects
National Farmworker Health Conference (May 22) - Presentation
Immunization Update, August 2006
ACIP Provisional recommendations for use of quadrivalent HPV vaccine
On August 14, CDC posted provisional recommendations for use of
HPV vaccine.
Routine vaccination with three doses of quadrivalent HPV vaccine is recommended for females 11–12 years of age. The vaccination series can be started in females as young as 9 years of age.
Friends Network
Produces "The Funletter," a national activities letter for kids and families living with cancer.
Address: c/o Kenon Neal
PO Box 4545
Santa Barbara, CA 93140
Chariots of Hope
A chariot of Hope, a nonprofit organization, has been established for the purpose of collecting used wheelchairs. The donated wheelchairs are then repaired and distributed, at no cost to the recipient, to those in need
National Children's Cancer Society
The mission of The National Children's Cancer Society is to improve the quality of life for children with cancer by promoting children's health through financial and in-kind assistance, advocacy, support services, and education.
Relief for Life
Description: Relief For Life is a not-for-profit organization dedicated to helping the disabled individuals by supplying wheelchairs and other ambulatory aides and devices to improve their lifestyle. Our mission of enabling disabled individuals to lead fuller lives is really all about empowering people so that they may live with a degree of satisfaction and dignity.
Center for Medicare and Medicaid
The Centers for Medicare & Medicaid Services (CMS) is a Federal agency within the U.S. Department of Health and Human Services. Programs for which CMS is responsible include Medicare, Medicaid, State Children's Health Insurance Program (SCHIP), HIPAA, and CLIA.
Childhood Leukemia Foundation (CLF)
CLF provides financial support to eligible families to help cover everything from rent, mortgage payments, food and utility bills, transportation to treatment, and other critical expenses.